
Vol.64, No.6 |
Science and Technology of
Energetic Materials
Vol.64, No.6, 2003 (334)
|
Contents
Papers: |
 |
Synthesis of alkaline-earth metal picrates |
 |
|
Makoto Matsukawa, Takehiro Matsunaga, Masatake Yoshida, and Shuzo Fujiwara |
227 |
| [Abstract]
Picric acid is known to react with metals to form highly unstable metallic picrates, which are known to have been involved in serious explosive accidents. In this study, alkaline-earth metal picrates of magnesium, calcium, strontium and barium salts are synthesized. Then, their thermodynamic and explosive properties such as initiation sensitivity are examined.
The decomposition of alkaline-earth metal picrates begins at a higher temperature than that of picric acid. The heat of decomposition of alkaline-earth metal picrates was found to be lower than that of picric acid. Alkaline earth-metal picrates contain crystalline H2O, which dehydrates stepwise with increasing temperature. Experimental results show that the amount of crystalline H2O in picrate in metastable phase is 9.2 - 9.9 H2O for Mg-picrate, 10.4 - 10.7 H2O for Ca-picrate, 5.0 - 5.1 H2O for Sr-picrate, and 5.9 - 6.4 H2O for Ba-picrate. However, the respective picrates changed to the stable form which contained the following amounts of crystalline water such as 6.5 H2O for Mg-picrate, 4.8 H2O for Ca-picrate, 4.0 H2O for Ba-picrate (Sr-picrate was not changed). The dehydration of crystalline water occurred between room temperature (about 298 K) and 480 K. Alkaline-earth metal picrates were dehydrated by heating in a vacuum at 473 K under 133 Pa. The activation energies at the initiation stage of exothermic decomposition are 125.6 kJ for Mg-picrate, 140.3 kJ for Ca-picrate, 171.3 kJ for Sr-picrate, and 257.7 kJ for Ba-picrate. Drop hammer test results show that Sr-picrate and Ba-picrate are more sensitive than picric acid.
|
|
 |
Study on the spontaneous ignition of cellulose nitrate
Effect of the type of storage atmosphere (I) |
 |
|
Katsumi Katoh, Lu le, Mamoru Itoh, Mitsuru Arai, and Masamitsu Tamura |
236 |
| [Abstract]
The effects of the type of storage atmosphere used on the spontaneous ignition of cellulose nitrate (NC) were investigated. Nitrogen dioxide, nitric oxide, oxygen, nitrogen, and dried air were used as storage atmospheres. Exothermic degradation was observed to be acceleratd in oxygen. In contrast, no degradation was observed with nitrogen. These results indicate that exothermic degradation by oxygen is related to spontaneous ignition. In addition, when low concentrations of nitrogen dioxide were used, there was no exothermic degradation. These results suggest that nitrogen dioxide contributes little to the generation of heat. |
|
 |
Blast wave parameters of PETN with silicon rubber binder |
 |
|
Dongjoon Kim, Yoshio Nakayama, Tomoharu Matsumura, Kunihiko Wakabayashi, Atsumi Miyake, Terushige Ogawa, and Masatake Yoshida |
241 |
| [Abstract]
The present study focuses on the blast wave parameters of pentaerythritol tetranitrate (PETN) with silicon rubber (SR) as a binder. It is able to change the mass, the shape and density of explosives. In this study, in order to confirm the effect of the binder on the strength of blast waves, the mixing ratio was changed as PETN/SR=50/50, 70/30, 100/0 wt.%. Blast wave pressures were measured in free air and on a rigid surface. The experimental results indicate that the PETN/SR explosives can detonate even only 1g of PETN/SR=70/30 wt.%. In addition, the mixing ratio was found not to affect the blast waves parameters, which indicates that the silicon rubber had no significant effect on the blast wave. Both the experimental and numerical results indicate that the blast wave parameters can be estimated to a sufficient degree of accuracy based only on the PETN mass. |
|
 |
Determination of JWL parameters from underwater explosion test for ideal and non-ideal explosives |
 |
|
Hideki Hamashima, Yukio Kato, You Nadamitsu, and Shigeru Itoh |
248 |
| [Abstract]
State of detonation products is described by various types of equations of state. Jones-Wilkins-Lee (JWL) equation of state is widely used because of its simplicity. JWL equation of state contains parameters determined by the cylinder expansion test. We obtained these parameters through the method of characteristics applied to the configurations of underwater shock waves of cylindrical explosives. The numerical results obtained by using the JWL parameters determined by the underwater explosion test are compared with the experimental results. Good agreement between the numerical and experimental results is confirmed in the case of the ideal and non-ideal explosives. |
|
Letter:
 |
Study on the spontaneous ignition of cellulose nitrate
Preparation and hazard evaluation of 1-O-methyl-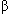 -d-glucopyranoside-2,3,4,6- tetranitrate as model compound of cellulose nitrate -d-glucopyranoside-2,3,4,6- tetranitrate as model compound of cellulose nitrate |
 |
|
Katsumi Katoh, Lu le, Mitsuru Arai, and Masamitsu Tamura |
254 |
| [Abstract]
Preparation and hazard evaluation of 1-O-methyl-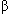 -d-glucopyranoside-2,3,4,6-tetranitrate(1) was performed in order to clarify the mechanism of spontaneous ignition of cellulose nitrate. The white crystal was obtained by reaction between 1-O-methyl- -d-glucopyranoside-2,3,4,6-tetranitrate(1) was performed in order to clarify the mechanism of spontaneous ignition of cellulose nitrate. The white crystal was obtained by reaction between 1-O-methyl-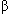 -d-glucoside and fuming nitric acid in chloroform. The white crystal was identified as 1 using elemental analysis, FT-IR spectrum, and melting-point by SC-DSC. Additionally, hazard evaluation was performed by friction test, drop hammer test, and electrostatic sensitivity test. It was confirmed that the sensitivities of 1 was not too high to treat as model compound for cellulose nitrate. -d-glucoside and fuming nitric acid in chloroform. The white crystal was identified as 1 using elemental analysis, FT-IR spectrum, and melting-point by SC-DSC. Additionally, hazard evaluation was performed by friction test, drop hammer test, and electrostatic sensitivity test. It was confirmed that the sensitivities of 1 was not too high to treat as model compound for cellulose nitrate. |
|
|
|
![]()